Abstract
Introduction: Compared to imatinib, second and third generation tyrosine kinase inhibitors (TKIs) achieve deeper and faster responses increasing the number of patients with chronic myeloid leukemia (CML) in treatment-free remission (TFR). However, they may raise the thrombotic risk. While nilotinib has been associated with accelerated atherogenesis, the prothrombotic mechanisms of ponatinib remain unclear, and may include von Willebrand Factor (VWF)-mediated platelet adhesion developing thrombotic microangiopathy. Better understanding of ponatinib thrombogenic mechanisms, a key therapy in patients with refractory CML, may provide a basis for its potential prevention.
Material and methods: Patients with chronic phase CML treated with imatinib 300-400 mg once daily (QD), nilotinib 400 mg QD-300 mg twice daily (BID), ponatinib 30-45 mg QD, or in TFR, as well as healthy blood donors (sex and age matched) from Spanish GELMC hospitals (n≥20 per group) were recruited. Peripheral blood samples were collected to obtain platelet-rich plasma for platelet activation assays (PFA-100, P-selectin expression and fibrinogen binding under stimulation with different agonists by flow cytometry, p-Syk levels by western-blot); and platelet-poor plasma to measure VWF: Ag, VWF:Collagen-Binding (VWF:CB), ADAMTS13 activity; cell-free DNA (cfDNA) and citrullinated histone 3 (citH3-DNA) as markers of neutrophil extracellular traps (NETs); platelet factor 4 (PF4); and chemokines, cytokines and proteins involved in coagulation and endothelial activation by Luminex.
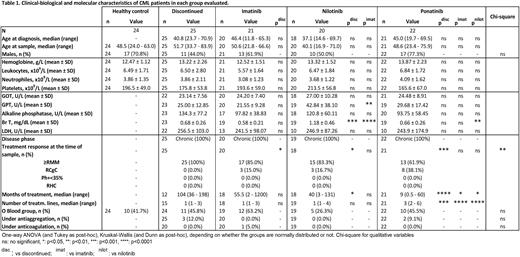
Results: Patients were comparable in age, sex and biological parameters, with no differences in the degree of molecular response in those under TKI treatment. As expected, patients under treatment with ponatinib had been exposed to more previous treatment lines and shorter exposure time to this TKI (Table 1). Our results showed plasma VWF:Ag levels were significantly elevated in ponatinib-treated CML patients compared to controls and other TKIs (p<0.01 and p<0.05, respectively). In fact, VWF:CB levels were also higher with ponatinib vs. controls (p<0.05). Moreover, ponatinib caused a reduction in ADAMTS13 activity, the main regulator of VWF function (p<0.05). Regarding NETs markers, we found that imatinib and ponatinib therapy raised cfDNA levels (p<0.001 and p<0.01 respectively), but only ponatinib elevated NETosis-specific marker (citH3-DNA) (p<0.05) in plasma.
Primary hemostasis assays showed that most ponatinib-treated patients had prolonged closure time Col/Epi (PFA-100 >300s) (p<0.001), whereas imatinib and nilotinib therapies did not change it. Compared to controls and other TKIs, platelets from ponatinib patients displayed decreased (50%) fibrinogen binding and P-selectin exposure following TRAP-6 and collagen-related peptide (CRP) stimulation, as well as decreased phosphorylation of Syk, suggesting defective GPVI activation pathway. However, no changes in plasma PF4 levels were observed comparing ponatinib with controls or other groups, which could indicate that platelet hyporeactivity under ponatinib treatment is not caused by in vivo activation in circulation. Interestingly, plasma samples from nilotinib patients had reduced PF4 levels compared to other groups, even with controls (p<0.05).
Luminex analysis showed greater soluble E-selectin and thrombomodulin levels in patients undergoing treatment with imatinib and ponatinib compared to controls, but there were statistical differences only for E-selectin (p<0.01 and p<0.05, respectively). Importantly however, plasma samples from ponatinib patients presented almost twice higher tissue factor (TF) levels (p<0.001), a potent procoagulant factor involved in arterial and venous thrombosis, while other TKIs had no impact on plasma levels of this protein.
Conclusions: Platelets from CML patients under treatment with ponatinib are hyporeactive indicating that antiplatelet therapy might not help prevent thrombotic events in these patients. Our results also suggest that the vaso-occlusive events of ponatinib are mainly due to endothelial damage rather than platelet dysfunction, with a critical role for thromboinflammatory markers such as VWF, NETs and TF. Indeed, plasma TF levels were remarkably elevated in ponatinib-treated patients vs. controls and other TKIs, pointing out TF inhibitors as a potential adjuvant therapy.
Disclosures
García Gutiérrez:Roche: Research Funding; BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Pérez-Encinas:Janssen: Consultancy. Vallansot:Incyte: Consultancy. Giraldo:ZeroLMC Foundation: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Acindes: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria. Ferrer Marin:Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal